The role of Early Head CT following the OHCA survival: results based on patients enrolled in RIAC
- 3/2019-Ottobre
- ISSN 2532-1285
- https://doi.org/10.23832/ITJEM.2019.039
Floriana Mancini1, Stefano Gandolfi2, Andrea Marudi3, Anna Vesely4, Giulio Righini5, Alberto Canalini6, Francesco Oddolini6, Claudia Cremonini7, Elisabetta Bertellini8, Geminiano Bandiera9
1. Emergency Department, Ospedale Civile Baggiovara, Modena, Italy
2. Italian Resuscitation Council Foundation, Bologna, Italy
3. Neurological Intensive Care Unit, Ospedale Civile Baggiovara, Modena, Italy
4. PhD Student, Università degli Studi di Padova, Padova, Italy
5. Emergency Department, Ospedale Civile Baggiovara, Modena, Italy
6. 118 Emergency System Department, AUSL Modena, Italy
7. Director of 118 Emergency System Department, AUSL Modena, Italy
8. Director of Neurological Intensive Care Unit, Ospedale Civile Baggiovara, Modena, Italy
9. Director of Emergency Department, Ospedale Civile Baggiovara, Modena, Italy
Abstract
Background
A multimodal approach is required to perform early neuro prognostication after cardiac arrest (CA). The objective of this study was to determinate the prognostic value of a positive early head computed tomography (EHCT) for anoxic-ischemic cerebral insult and its impact on 30-day survival rate.
Methods
The retrospective study included all out-of-hospital cardiac arrest (OHCA) events, anonymously recorded with the Italian Registry of Cardiac Arrest (RIAC), occurred between January 1st, 2015 and December 31st, 2018 in which EHCT was performed within 2 hours upon ER arrival.
Results
The characteristics of 156 patients were collected. 126 patients (81%) had an EHCT before admission to ICU, of these: n° 89 had a negative CT, n° 30 had a positive CT for anoxic-ischemic cerebral insult and n° 7 were excluded due to subarachnoid hemorrhage. Our data highlighted a positive association between EHCT and death, but specificity was 89% and sensitivity 38% meaning that the presence of brain injury was able to correctly predict 38% of the deaths and 89% of the positive outcomes.
Conclusion
EHCT seems able to classify patients that will survive but tends to produce a high number of false negatives (62%). Therefore, in our cohort, it is insufficient in isolation to definitively prognosticate.
Keywords
Computed Tomography, Out-Of-Hospital Cardiac Arrest, Outcomes, 30-days-survival, Prognostication
Introduction
Out-of-hospital cardiac arrest (OHCA) is a leading cause of death in Europe with an overall survival rate of 2.6-9.9%1,2. Across Europe, the annual incidence of OHCA is 84 cases per 100.000 inhabitants, among these, CPR is attempted in 49 patients. Data on survival to 30 days or to hospital discharge showed that the percentage ranged from 1.1% and 30.8%; with a bystander witnessed arrest, a suspected cardiac cause, and an initial recorded shockable rhythm the hospital survival rates ranged from less 6% up to 55%3. Prompt delivery of high-quality CPR can improve survival outcome and neurologic recovery in OHCA4-6 but Italian data on CA are incomplete and fragmented due to the availability of only local reports while a national representation is lacking7. The best way to describe the epidemiology of a disease is to create a registry in which the disease is reported because it can describe changes over time in incidence, survival and various mode of treatments3. Thus, the Italian Resuscitation Council (IRC) has developed a National Registry of CA called RIAC – (Registro Italiano Arresti Cardiaci – Italian Registry of Cardiac Arrest)8 NCT03220269 which is a free web registry based on Utstein style data9,10 for all EMS and ICU departments. This registry has been collecting cases since 2014 with the aim to reduce the fragmentation of data. RIAC records the full medical history of patients (≥ 18 years old) from the onset of CA through CPR (both pre-hospital and in-hospital)11 and post-resuscitation care. Post-resuscitation data include information on patients’ management and processes of care to assess the quality of interventions and their effects on outcome. Therefore, specific patients’ physiologic data are included within the database along with results of neurological examinations and biomarkers. The two known computed tomography (CT) signs associated with ischemic brain damage after OHCA are loss of boundary (LOB) between grey matter and white matter and cortical sulcal effacement. Both signs are expected to be used as early prognosticators in OHCA survivors, but the capability of imaging studies for outcome prediction have been insufficiently investigated12. Thus, the objective of this retrospective study was to determine the prognostic value of a positive Early Head CT – EHCT for anoxic-ischemic cerebral insult and its impact on survival at 30 days after OHCA.
Methods
1.1 Study design
The study includes all OHCA events, anonymously recorded in RIAC, occurred between January 1st, 2015 and December 31st, 2018 within the province of Modena. The province of Modena covers an area of 2.688 square kilometers divided into 47 municipalities and counts a resident population of 701.896 people13. Inclusion criteria were age ≥ 18 years old and confirmed CA in which there was a resuscitation attempt defined as chest compression only or full CPR with/or without defibrillation and ROSC. For patients having more than one CA during the same hospitalization, only the out-of-hospital arrest was included. Management of ROSC patients in ER followed specific procedures and treatments guided by a local ROSC protocol developed according to the European Resuscitation Council (ERC)-ESICM guidelines for post-resuscitation care14.
1.2 Patients selection
Consulting RIAC we found 408 patients who suffered of an OHCA from January 1st, 2015 to December 31st, 2018. Out of these 408, 252 arrived dead at hospital admission, while 156 were successfully resuscitated and transported to hospital. Of these 156, 126 had an EHCT within 2 hours after admission to ER (see Figure 1). We used non-contrast head CT acquired with 5 mm slices. The main CT findings were reported by certified radiologists as:
- Anoxic-ischemic cerebral insult, which appears as a reduction in the depth of cerebral sulci and an attenuation of the grey matter to white matter differentiation.
- Subarachnoid hemorrhage.
- Negative EHCT.
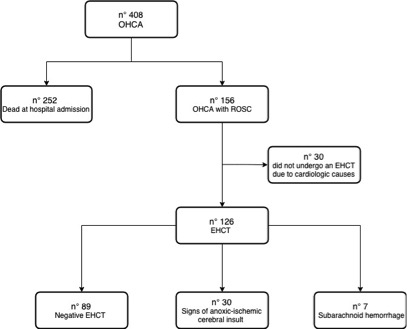
Figure 1. Flow chart demonstrating the patients’ selection process from RIAC
1.3 Statistical analysis
Baseline characteristics are reported as mean ± SD, median [IQR] or percentage (%), as appropriate. To evaluate the risk of death based on different risk factors we calculated the Hazard Ratio (HR) using a Cox regression model; percent survival was represented by Kaplan-Meier curves. A p-value <0.05 was accepted as statistically significant. The rate of unfavorable outcomes was compared between those who were negative for ischemic brain injury vs those who were positive. The sensitivity and specificity of each EHCT was calculated. Statistical analysis was performed with Stata 13.0.
Results
2.1 Overall population characteristics
The characteristics of the 156 patients arrived with ROSC are summarized in Table 1. The mean and median age are both 70 years. The majority of patients are male (67%), had a witnessed CA (64%), arrived during the day shift (74%), did not report chest pain before (74%), did not have a known heart disease (59%), and were autonomous before (67%). 126 patients (81%) had an EHCT before admission to ICU, and 61 (39%) had a CAG immediately after ER evaluation. 90% of patients (n° 141) were admitted to ICU. 30 days after ROSC, 83 patients (53%) were dead (mean 7 days, median 4 days), whereas 73 patients were alive. Initial rhythms of presentation were VF/VT in 60% of cases (n° 93), PEA was present in 27% of cases (n° 42) and asystole in 13% (n° 21). The population was then divided into three groups according to the cardiac arrest causes: cardiogenic, non-cardiogenic15 and unknown etiology. Among cardiogenic group (53% of patients, n° 82), acute coronary syndrome (ACS) is the most common cause (63%), whilst respiratory failure (32%), sepsis (23%) and electrolyte abnormality (18%) represent the three main causes of non-cardiogenic CAs (40% of patients, n° 62).
Characteristics | Values |
Mean age ± SD (years) | 70 ± 14 |
Median age (years) | 70 |
Male sex n/N (%) | 104/156 (66.7%) |
Witnessed CA n/N (%) | 100/156 (64.1%) |
Night shift n/N (%) | 40/156 (25.6%) |
Reported chest pain before CA n/N (%) | 40/156 (25.6%) |
Known Heart Disease before CA n/N (%) | 63/156 (40.7%) |
Severe disability before CA n/N (%) | 51/156 (33.1%) |
Admission to ICU n/N (%) | 141/156 (90.4%) |
Mean length of stay in ER ± SD (minutes) | 55 ± 28 |
Mean length of hospital stay ± SD (days) | 18 ± 22 |
Type of CA causes n/N (%) Cardiogenic causes Non-cardiogenic causes Unknown causes |
82/156 (52.6%) 62/156 (39.7%) 12/156 (7.7%) |
Causes of Cardiogenic CA n/N (%) Acute Coronary Syndrome – ACS Cardiomyopathy AV block Channelopathy Myocarditis |
52/82 (63.4%) 16/82 (19.5%) 5/82 (6.1%) 5/82 (6.1%) 4/82 (4.9%) |
Causes of Non-Cardiogenic CA n/N (%) Respiratory failure Sepsis Electrolyte abnormality Subarachnoid hemorrhage Anaphylactic shock Pulmonary embolism Trauma Aortic abdominal aneurism rupture |
20/62 (32.3%) 14/62 (22.6%) 11/62 (17.7%) 7/62 (11.3%) 4/62 (6.5%) 4/62 (6.5%) 1/62 (1.6%) 1/62 (1.6%) |
Initial Rhythm n/N (%) VF/VT PEA Asystole |
93/156 (59.6%) 42/156 (26.9%) 21/156 (13.5%) |
Early Head CT n/N (%) Negative Signs of anoxic-ischemic brain injury Subarachnoid Hemorrhage | 126/156 (80.8%) 89/126 (70.6%) 30/126 (23.8%) 7/126 (5.6%) |
Emergency coronary angiography – CAG n/N (%) | 61/156 (39.1%) |
Death at 30 days n/N (%) | 83/156 (53.2%) |
Mean period before death ± SD (days) | 7 ± 6 |
Median period before death (days) | 4 |
Table 1. Baseline characteristics of the OHCA population admitted with ROSC
Table 2 shows the differences between CAs etiologies (excluding the CAs of unknown causes). Cardiogenic CAs has VF/VT as predominant rhythm of presentation with a frequency of 85%, while PEA is more prevalent in non-cardiogenic CAs with a frequency of 44% among this group. Asystole was recorded in 23% of the patients of the non-cardiogenic CAs group and only in 4% of the patients of the cardiogenic CAs group.
| Cardiogenic CA | Non-Cardiogenic CA |
Mean age ± SD (years) | 69 ± 13 | 71 ± 15 |
Male sex n/N (%) | 62/82 (75.6%) | 33/62 (60.0%) |
Initial Rhythm VF/VT n/N (%) PEA n/N (%) Asystole n/N (%) |
79/82 (85.4%) 9/82 (11.0%) 3/82 (3.7%) |
21/62 (33.9%) 27/62 (43.6%) 14/62 (22.6%) |
Emergency coronary angiography – CAG n/N (%) | 57/82 (69.5%) | 3/62 (4.8%) |
Death at 30 days n/N (%) | 35/82 (42.7%) | 37/62 (59.7%) |
Table 2. Differences between cardiogenic and non-cardiogenic CA population
2.2 EHCT findings
Subsequently, we compared patients with signs of anoxic-ischemic brain injury and patients with negative EHCT. Those who did not have an EHCT (n° 30) or had subarachnoid hemorrhage (n° 7) were excluded. Out of the remaining 119 patients, 63 (53%) died in the first 30 days; in particular, 25% patients died within 5 days, while 50% died within 16 days. We studied predictors of survival at 30 days individually, using Cox proportional hazard model to analyze EHCT results (positive for signs of anoxic-ischemic brain injury vs negative), age, sex, witnessed CA, shift (night vs day), cause (cardiogenic vs non-cardiogenic or unknown) and initial rhythms (FV/TV vs PEA or asystole). The choice of such model is justified by preliminary graphical analysis, which suggests that the initial assumption of proportional hazards is met. With a confidence level of 0.05, univariate analyses showed that a witnessed CA (p=0.021) and VF/VT as initial rhythms of presentation (p=0.004) represent positive factors of survival, while the presence of signs of anoxic-ischemic brain injury (p<0.001) and older age (p<0.001) are negative outcome predictors. Table 3 shows the HRs of the above-mentioned predictors, and Figure 2 shows Kaplan-Meier survival estimates according to the presence of sings of anoxic-ischemic cerebral insult.
| Hazard Ratio (95% CI) | p |
Age | 1.04 (1.02-1.07) | <0.001 * |
Age ≥ 75 | 2.72 (1.63-4.56) | <0.001 * |
Male sex | 0.64 (0.39-1.05) | 0.078 |
Witnessed CA | 0.56 (0.34-0.92) | 0.021 * |
Night shift | 1.15 (0.66-1.98) | 0.620 |
Cardiogenic CAa | 0.62 (0.37-1.02) | 0.058 |
Initial Rhythms FV/TVb | 0.49 (0.30-0.80) | 0.004 * |
Signs of anoxic-ischemic cerebral insult at EHCTc | 2.70 (1.62-4.52) | <0.001 * |
Table 3. Univariate survival hazard models at 30 days
a Cardiogenic CA compared to Non-Cardiogenic CA and Unknown CA.
b Initial Rhythms FV/TV compared to PEA and Asystole.
c Patients who did not have an EHCT or had subarachnoid hemorrhage are excluded.
* = statistical significance with a 0.05 confidence level
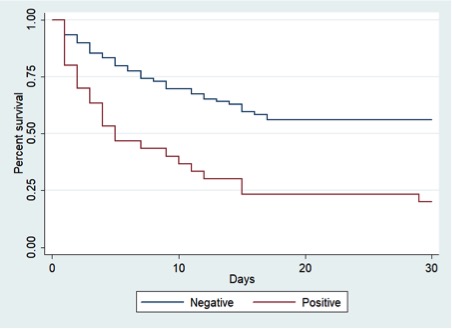
Figure 2. Kaplan-Meier plot of patients’ survival at 30 days comparing negative EHCT vs positive EHCT for signs of anoxic-ischemic cerebral insult
In order to evaluate the most important predictors of survival at 30 days, a multivariate analysis (see Table 4) was performed using Cox proportional hazard model.
| Hazard Ratio (95% CI) | p |
Age | 1.05 (1.02-1.07) | <0.001 * |
Male sex | 0.77 (0.46-1.29) | 0.325 |
Witnessed CA | 0.64 (0.38-1.07) | 0.092 |
Night shift | 1.15 (0.67-2.02) | 0.616 |
Cardiogenic CAa | 1.00 (0.55-1.82) | 1.000 |
Initial Rhythms FV/TVb | 0.43 (0.24-0.79) | 0.006 * |
Signs of anoxic-ischemic cerebral insult at EHCTc | 2.14 (1.25-3.67) | 0.005 * |
Table 4. Multivariate survival hazard model at 30 days
a Cardiogenic CA compared to Non-Cardiogenic CA and Unknown CA.
b Initial Rhythms FV/TV compared to PEA and Asystole.
c Patients who did not have an EHCT or had subarachnoid hemorrhage are excluded.
* = statistical significance with a 0.05 confidence level.
With a confidence level of 0.05, the significant risk factors are the presence of signs of anoxic-ischemic brain injury (p=0.005) and age (p<0.001), while the only factor significantly associated with survival is VF/VT as initial rhythms of presentation (p=0.006). All other covariates being equal, the hazard rate for patients with positive EHCT is 2.14 times higher than the hazard rate of other patients. Analogously, the hazard rate increases of 5% for each year of age and decreases of approximately 60% for patients with VF/VT as initial rhythms. The witness of the CA no longer results to be a significant factor. Finally, we studied how EHCT might fit into the framework of prognostication by analyzing its ability to predict death at 30 days (see Table 5).
| Dead | Alive | Tot. |
Positive EHCT | True positives 24 (38%) | False positives 6 (11%) | 30 |
Negative EHCT | False negatives 39 (62%) | True negatives 50 (89%) | 89 |
Tot. | 63 (100%) | 56 (100%) | 119 |
Table 5. Contingency table of EHCT results and death at 30 days
80% of patients with positive EHCT for post anoxic brain injury and 44% of patients with negative EHCT died within 30 days. The positive likelihood ratio LR+ is 3.56, indicating a positive association between EHCT and death. The sensitivity is 0.381, hence 38% of patients that died had a positive EHCT. The specificity is 0.893, meaning that 89% of patients that survived had a negative EHCT. In other words, the presence of signs of anoxic-ischemic brain injury was able to correctly predict 38% of the deaths and 89% of the positive outcomes, while 62% of patients that died had negative EHCT (false negatives) and 11% of patients that survived had positive EHCT (false positives).
Discussion
In this retrospective study, OHCAs were prevalent in males with a mean and median age of both 70 years. The 64% of all cardiac arrests were witnessed and had a VF/TV as onset rhythm. Cardiogenic causes of CA were the most frequent category observed with acute coronary syndrome as leading etiology whereas non-cardiogenic were less represented. After ROSC, prior to ICU admission, 126 patients underwent an EHCT. Of these, for n° 89 CT did not find signs of brain injury, instead anoxic brain injury was diagnosed in n° 30 patients, finally n° 7 were excluded due to subarachnoid hemorrhage16,17. In this cohort of 119 patients, 63 died within 30 days, most of them within 16 days, pointing out that the OHCA is still a major cause of mortality. Neuroprognostication in post-cardiac arrest patients is clinically challenging and reliable strategies for timely prognostication are a critical component of any cardiac arrest system of care. The earliest time for prognostication in post-ROSC patients treated with TTM is 72 hours after return to normothermia18,19. The four key components of post-cardiac arrest syndrome had been identified as (1) post-cardiac arrest brain injury, (2) post-cardiac arrest myocardial dysfunction, (3) systemic ischemia/reperfusion response, and (4) persistent precipitating pathology20. Among these, brain injury is the cause of death between 54% to 68% after OHCA and our data are in line with these findings21,22. The multivariate analysis for 30-day survival showed that the factors that appear to be strongly associated with mortality were a positive EHCT for brain injury (p=0.005), age (p<0.001) and onset rhythm (p=0.006). All other covariates, gender, witnessed CA, night shift and cardiogenic causes of cardiac arrest were no longer significant factors. Previous studies highlighted that the onset rhythms with the higher chance of outliving are the shockable ones23 which are the most frequent in cardiogenic CAs compared to non-cardiogenic CAs: in our cohort 85.4% vs 33.9% of prevalence of VF/VT respectively. Within our data, the hazard rate correlated, decreased of approximately 60% for patients with shockable initial rhythms. An anoxic-ischemic cerebral insult after ROSC in OHCA patients is linked to a lower survival rate at 30 days24. Therefore we wanted to study how exactly the CT findings are related to death after OHCA. Our data highlighted a positive association between EHCT and death, but specificity was 89% and sensitivity 38% meaning that the presence of brain injury was able to correctly predict 38% of the deaths and 89% of the positive outcomes. In conclusion, EHCT seems able to classify patients that will survive, but tends to produce a high number of false negatives (62%), therefore, it is insufficient in isolation to definitively prognosticate.
3.2 Consideration and current evidences
The ILCOR international guidelines identified different studies using imaging as a prognostic tool but all of them were prone to selection bias and had low or very low-quality evidence. Hence there is not a standard for the critical outcome of survival with unfavorable neurologic status or death at discharge, or neurologic status or death at 180 days. The task force suggested the use of clinical criteria alone before 72 h after ROSC to estimate prognosis and a multiple modality of testing (clinical exam, neurophysiological measures, imaging or blood markers) to estimate prognosis instead of relying on single tests or findings17. Prior CT studies focused on attenuation of the gray-white matter (GM/WM) interface quantitatively measured as the ratio (GWR) and cerebral edema12,20,25-27. Inamasu et al demonstrated that a time window of £ 20 min vs > 20 min from CA to ROSC prognosticate as favorable aftermath in association with a better CPC (1-2). In particular, they analyzed the loss of boundary sign and found out that a positive LOB on CT was predictive of unfavorable outcome with 81% sensitivity and 92% sensibility. But they excluded non-witnessed cardiac arrest in order to define a better window period. The CA to ROSC interval regarding the Utstein recommendation9 relies on many variables such as the memory of witnesses25 and some of the others are impossible to record or they are unlikely to be recorded9. This state-of-art scenario by Inamasu et al is not possible in our realty therefore the discrepancy of data are obviously. When combination of different tools is combined better prediction of outcome are possible. In fact, the combination of CT and the neuro specific enolase – NSE had been studied by Lee et al. The analysis of 119 patients undergoing both brain CT and NSE at 48 h indicated that CT and NSE improved the sensitivity up till 78.6% compared to either alone (48.6%, 62.9%). Sensitivity was found similar in our group but lower (38%). Metter et al according to their study suggested that the average GWR can estimate risk of mortality for patients after CA prior to treatment with or without hypothermia but does not report any data concerning sensibility and specificity. Fugate et al instead studied the cerebral edema with malignant EEG finding a statistical association in hypothermic and non-hypothermic group but also bringing up a false-positive rate of NSE of 29.3%. Reynolds et al highlight the bias involved in decision-making regarding whether to perform an EHCT, but there are no data available about sensibility and specificity as well.
3.3 Limitations
The study has many limitations. The decision-making process regarding when to obtain an EHCT was influenced by a patient’s premorbid status and therefore there is no exception within the ROSC protocol. Among our 156 patients, 30 did not undergo an EHCT due to cardiologic causes. Also, this study spanned over four years and due to the continuous shifting of healthcare professionals, data such as CPCs, variable time to HCT, bystander CPR were lacking or not complete.
Overall Conclusion
EHCT images showing an anoxic-ischemic cerebral insult after CA are linked to a lower survival rate at 30 days. Our data underlines the importance of EHCT in patient resuscitated from out-of-hospital cardiac arrest as a tool to obtain a primary indication for survival but EHCT tends to produce a high number of false negatives (62%).
Bibliography
- Daya MR, Schmicker RH, Zive DM et al. Out-of-hospital cardiac arrest survival improving over time: Results from the Resuscitation Outcomes Consortium (ROC). Resuscitation. 2015; 91:108–15. DOI: 10.1016/j.resuscitation.2015.02.003
- Wnent J, Masterson S, Grasner JT et al. EuReCa ONE-27 Nations, ONE Europe, ONE Registry: a prospective observational analysis over one month in 27 resuscitation registries in Europe-the EuReCa ONE study protocol. Scand J Trauma Resusc Emerg Med. 2015; 23:7. Epub 2015/05/01. DOI: 10.1186/s13049-015-0093-3
- Gräsner JT, Lefering R, Koster RW et al. EuReCa ONE-27 Nations, ONE Europe, ONE Registry: A prospective one-month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation. 2016 Aug;105:188-95. DOI: 10.1016/j.resuscitation.2016.06.004
- Kim S, Ahn OK, Jeong S. The effect of team-based CPR on outcomes in out of hospital cardiac arrest patients: A meta-analysis. February 2018 Volume 36, Issue 2, Pages 248-252. DOI: https://doi.org/10.1016/j.ajem.2017.07.089
- Abella BS. High-quality cardiopulmonary resuscitation: current and future directions. Curr Opin Crit Care 2016 Jun;22(3):218-24. doi: 10.1097/MCC.0000000000000296.
- Olasveengen TM, de Caen AR, Mancini ME et al. 2017 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations Summary. Circulation. 6 Nov 2017;136:e424–e440 https://doi.org/10.1161/CIR.0000000000000541
- Kette F, Pellis T, Pordenone Cardiac Arrest Cooperative Study Group (PACS). Increased survival despite a reduction in out-of-hospital ventricular fibrillation in north-east Italy. Resuscitation 2007;72:52–8. DOI:10.1016/j.resuscitation.2006.05.022
- Ristagno G, Semeraro F, Radeschi G et al. The “Italian Registry of Cardiac Arrest – RIAC”, a National achievement to portrait the Italian reality and to contribute to the wider European vision by “EuReCa”. Resuscitation 2014 Dec;85(12):e193-4. DOI: https://doi.org/10.1016/j.resuscitation.2014.09.015
- Perkins GD, Jacobs IG, Nadkarni VM et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee. Circulation 2015; 132: 1286–300. DOI: 10.1161/CIR.0000000000000144
- Cummins RO, Chamberlain D, Hazinski MF et al. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital ‘Utstein style’. A statement for healthcare professionals from the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Australian Resuscitation Council, and the Resuscitation Councils of Southern Africa. Resuscitation 1997;34:151-83. DOI: https://doi.org/10.1016/S0300-9572(97)01112-X
- Radeschi G, Mina A, Berta G et al. Incidence and outcome of in-hospital cardiac arrest in Italy: a multicentre observational study in the Piedmont Region. Resuscitation 2017 Oct;119:48-55. Epub 2017 Jun 24. DOI: 10.1016/j.resuscitation.2017.06.020
- Inamasu J, Miyatake S, Suzuki M et al. Early CT signs in out-of-hospital cardiac arrest survivors: Temporal profile and prognostic significance. Resuscitation, Volume 81, Issue 5, 534 – 538. DOI: https://doi.org/10.1016/j.resuscitation.2010.01.012
- Canalini A, Cremonini C, Oddolini F et al. Out-Of-Hospital Cardiac Arrest: an overview of the 2017 cases in the province of Modena. Italian Journal of Emergency Medicine. February 2019, Vol 1.
- Nolan JP, Soar J, Cariou A et al. European resuscitation council and European society of intensive care medicine 2015 guidelines for post‐resuscitation care. Intensive Care Med 41:2039–205 DOI: 10.1007/s00134-015-4051-3
- Myat A, Song KJ, Rea T. Out-of-hospital cardiac arrest: current concepts. The Lancet, Volume 391, Issue 10124, 970-979. DOI:https://doi.org/10.1016/S0140-6736(18)30472-0
- Skrifvars MB, Parr MJ. Incidence, predisposing factors, management and survival following cardiac arrest due to subarachnoid haemorrhage: a review of the literature. Scand J Trauma Resusc Emerg Med. 2012; 20: 75. doi: 10.1186/1757-7241-20-75
- Arnaout M, Mongardon N, Deye N et al.Out-of-hospital cardiac arrest from brain cause: epidemiology, clinical features, and outcome in a multicenter cohort. Crit Care Med. 2015 Feb;43(2):453-60. doi: 10.1097/CCM.0000000000000722.
- Pothiawala S. Post-resuscitation care. Singapore Med J. 2017 Jul; 58(7): 404–407. doi: 10.11622/smedj.2017060
- Soar J, Clifton CW, Aibiki M et al. Part 4: Advanced life support 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 95 (2015) e71–e120. DOI: 10.1161/CIR.0000000000000273
- Nolan JP, Neumar RW, Adrie C et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. Resuscitation (2008) 79, 350—379
- Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med 2004;30:2126—8.
- Reynolds AS, Matthews E, Magid-Bernstein J et al. Use of Early Head CT following Out-of-Hospital Cardiopulmonary Arrest. Resuscitation. 2017 April ; 113: 124–127. doi:10.1016/j.resuscitation.2016.12.018.
- Fukuda T, Matsubara T, Doi K et al. Predictors of favorable and poor prognosis in unwitnessed out-of-hospital cardiac arrest with a non-shockable initial rhythm Intern Journal of Cardiology 2014 Volume 176, Issue 3, 910–915. DOI: 10.1016/j.ijcard.2014.08.057
- Brooks SC, Schmicker RH, Rea TD et al. Out-of-Hospital Cardiac Arrest Frequency and Survival: Evidence for Temporal Variability Resuscitation. 2010 February; 81(2): 175. DOI: 10.1016/j.resuscitation.2009.10.021
- Metter RB, Rittenberger JC, Guyette FX et al. Association Between a Quantitative CT Scan Measure of Brain Edema and Outcome After Cardiac Arrest. Resuscitation. 2011 September ; 82(9): 1180–1185. doi:10.1016/j.resuscitation.2011.04.001.
- Lee BK, Jeung KW, Lee HY et al. Combining brain computed tomography and serum neuron specific enolase improves the prognostic performance compared to either alone in comatose cardiac arrest survivors treated with therapeutic hypothermia. Resuscitation, Volume 84, Issue 10, 1387 – 1392. DOI: https://doi.org/10.1016/j.resuscitation.2013.05.026
- Fugate JE, Wijdicks EF, Mandrekar J et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol, 68: 907-914. doi:10.1002/ana.22133

