- Boccatonda A.
- Brief Report and Case Report
Lung ultrasound beyond the pleural line: a case of respiratory failure due to malignant mesothelioma
- 1/2019-Febbraio
- ISSN 2532-1285
- https://doi.org/10.23832/ITJEM.2019.009
Boccatonda A., D’Ardes D., Cocco G., Sanarica I., Schiavone C.
Internistic Ultrasound Unit, Department of Medicine and Science of Aging, “G. D’Annunzio” University, Chieti 66100, Italy
Abstract
Background: Lung ultrasound (LUS) exerts a relevant role in early evaluation of lung and pleural diseases, especially in the emergency setting. In particular, LUS has been increasingly employed to differentiate malignant from benign pleural masses.
Case Report: we report the case of a 85 years old caucasian male referred to our emergency department for the onset of dyspnea, fatigue and atypical chest pain. LUS showed a mass with mixed echogenicity and irregular contours at right hemitorax, near the diaphragmatic surface, suspected for malignant neoplasia. Subsequent chest computed tomography and histological examination confirmed the diagnosis of malignant mesothelioma.
Conclusions: Mesothelioma is a challenging diagnosis for physicians due to no specific symptoms and lack of a reliable biomarker. LUS is a non-invasive and reliable imaging modality allowing to detect early signs of asbestosis and mesothelioma development such as pleural effuson, pleural thickening and masses.
Keywords
mesothelioma, ultrasound, lung, pleura, mass.
Introduction
Lung ultrasound (LUS) exerts a relevant role in early evaluation of pulmonary and pleural diseases, especially in the emergency setting [1-5]. From the early works of Lichtenstein et al [6, 7], the semeiotic of LUS has become increasingly codified and standardized, allowing the recognition and the differential diagnosis of serious and lifethreating diseases, such as acute pulmonary edema, pleural effusion and pneumothorax [4, 8-10]. In particular, in recent years, pleural line and diaphragm motility evaluation have been employed as a quantitative method to monitor ventilatory function, for example as a predictive factor in patients subjected to mechanical ventilation [11, 12]. Moreover, a qualitative sonographic examination of the pleura and diaphragm can be performed, thus allowing the diagnosis of complex diseases such as neoplastic masses [13, 14].
Case Report
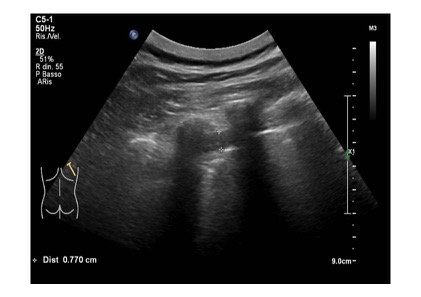
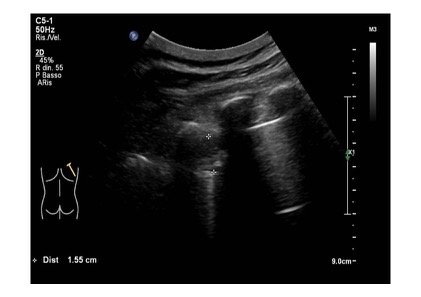
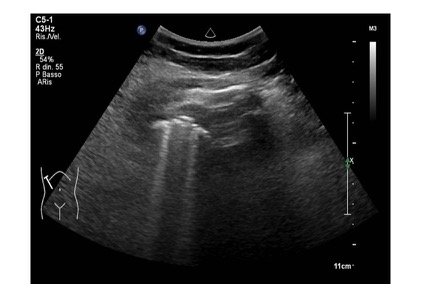
A 85 years old caucasian male cames to our emergency department for the onset of dyspnea, fatigue and atypical chest pain localized at the lower segment of right hemithorax. His medical hystory was characterized by essential hypertension leading to hypertrophic cardiomyopathy, pacemaker implantation and benign prostatic hypertrophy. At physical examination upper airways were pervious, with a respiratory rate of 32 acts /minute and a peripheral oxigen saturation of 87% (FiO2: 21%). Heart rate was regular with 86 bpm and blood pressure was 110/70 mmHg. He was drowsy on presentation, but without any focal neurological deficits. Blood gas analysis revealed an hypoxemic normocapnic respiratory failure, which was treated with oxygen supplementation. At the same time, a LUS was performed to ascertain the reasons responsible for respiratory failure. Pleural sliding was reduced in middle and lower parts of right hemitorax and in lower segments of left hemitorax. Pleural line was diffusely and irregularly thickened and jagged. At the lower segment of right hemitorax, a slight pleural effusion was evidenced. In addition, similar areas of loculated pleural effusion were found in the middle and apical segments of right hemitorax. Moreover, we detected a mass with mixed echogenicity and irregular contours at the base of right hemitorax, attached to the diaphragmatic surface, with a maximum size of 1.5 cm. A similar area was reported at apex of the right hemitorax. Few B lines spread asymmetrically (Fig 1,2,3).
Figure 1: Mid-apical scan showing pleural line diffusely and irregularly thickened and jagged, with some small loculated pleural effusion.
Figure 2: A mass with mixed echogenicity and irregular contours at the lower part of right hemitorax, attached to the diaphragmatic surface
Figure 3: Comet-tail artifacts (B lines) are generated at the interface between mass-compressed atelectatic lung and normal ventilated lung.
Following these sonographic findings, a chest computed tomography (CT) was performed showing in the apical segment of right hemitorax an opacity with net margins, and a pleural effusion layer, partially loculated, in the apical segment and along the margino-costal line in the right hemitorax. There was a parenchymal hypoventilation of the homolateral lower segment. Moreover, a pleural thickening at basal segment of right hemitorax (maximum thickness of 1.5 cm) and of the the great fissure were seen.
Subsequently, an ultrasound-guided biopsy of the apical pleural mass was performed, which confirmed the suspicion of malignant pleural mesothelioma (MPM). Later, the patient was assigned to thoracic surgeons to perform a thoracoscopy and to continue the diagnostic and therapeutic management.
Discussion
MPM is an aggressive tumor involving pleural surface displaying a median survival from 8 to 14 months from first diagnosis [15, 16]. MPM incidence is higher in men, probably due to occupational risk factors, and it increased over recent years [15-17]. Indeed, MPM is mainly related to asbestos occupational exposure [15, 17], but other less common risk factors are erionite, chest wall radiation and simian virus 40 [15, 18]. A typical triad of pleural effusion, dyspnea, and chest wall pain characterize patients affected by MPM in 60% of cases [17, 19, 20]. Other symptoms such as laryngeal nerve palsy or dysphagia, or superior vena cava obstruction syndrome and peritoneal involvement are due to tumor invasion and diffusion [15, 17]. Notably, many patients are asymptomatic, and mesothelioma can be incidentally detected on imaging [15]. In our patient, the clinical and anamnestic evaluation allowed early to address the diagnosis towards a neoplastic pleural disease, subsequently confirmed by histological examination as MPM. Indeed, our patient reported on his past history an exposure to several fine powders, including asbestos, since he worked as a mason.
First of all, LUS can detect sonographic signs of asbestosis in exposed subjects, such as irregular pleural surface with peripheral lung consolidation and diffuse bilateral interstitial syndrome with non-homogeneous distribution (pulmonary asbestosis) [13, 14]. Furthermore, sonographic findings suggesting MPM include pleural-based masses, pleural thickening >1 cm, nodular pleural thickening and diaphragmatic nodularity [13, 14, 21]. Mesothelioma pleural masses are hypoechoic lesions or with a mixed echo-pattern at ultrasound examinations, often related to a pleural thickening and surrounded by aerated lung [22]. Moreover, ultrasound has been demonstrated to be more accurate than chest CT scan for detection of cancer chest wall invasion [23]. Pleural effusion is a common finding in patients affected by MPM, that can be detected on ultrasound as an anechoic or hypoechoic space within the thorax. Ultrasound examination better characterizes the internal complexity of a pleural effusion in comparison with standard radiography and chest CT scan [24]. The contemporary presence of nodules or masses on the hemidiaphragm may represent metastatic foci, and it’s quite typical of a malignant pleural effusion [24-26]. Pleural effusion induces compressive atelectasis of the nearest lung, by reducing the air amount until reaching alveolar consolidation pattern. At the interface between atelectatic lung and normal ventilated lung, there is often an echogenic boundary from which comet-tail artifacts are generated. An ultrasound-guided thoracentesis may be performed for diagnostic or therapeutic purposes [20, 27, 28]. Ultrasound allows to select the safest insertion site, the best angle, and to measure the depth for needle insertion into a pleural effusion, thereby decreasing complication rate, including pneumothorax or injury to nearest organs [29-31]. Moreover, one of the biggest advantages to employ ultrasound for monitoring the positioning of device and checking the amount of pleural effusion is the reduction of radiation exposure due to x-ray controls and the greater easiness and speed of execution.
In our case, LUS evaluation showed a minimum amount of pleural effusion, especially detected with breathing changes (curtain sign alteration). Subsequently, the examination of anterior, lateral and posterior scans of the diaphragmatic profile showed the presence of solid mass attached to the diaphragmatic muscle of a maximum size of 1.5 cm. Furthermore, mid-apical scans showed additional asymmetric and irregularly distributed pleural nodules, with some small loculated pleural effusion. Subsequently, an ultrasound-guided biopsy of pleural masses was performed, thus confirming the suspicion of MPM. Even if clear data about ultrasound superiority still lack in compaison with CT guidance, ultrasound-guided biopsy has been demostrated to be a safe procedure to make diagnosis by using needle aspiration or a cutting biopsy needle, displaying a high sensitivity and a decreased patient radiation dose [32-34]. US-guided cutting-needle biopsy (CNB) and standard pleural biopsy (SPB) procedures provide adequate material for histological analysis in 90.7 and 93.0% of cases, respectively, while the combination of CNB and SPB significantly improve the sensitivity, NPV and diagnostic accuracy versus each technique alone [35].
Finally, subjects who report previous asbestos exposure or present with a pleural effusion should be carefully monitored [36]. LUS can be employed as an additional examination to monitor patients occupationally exposed to asbestos who have already performed CT scan and whose disease is detectable by ultrasound as well [13, 14].
Conclusions
Mesothelioma is a challenging diagnosis for emergency physician due to no specific symptoms and lack of a reliable biomarker. Radiological imaging should be performed in all patient with anamnestic and clinical suspicion of MPM. In particular, LUS is a non-invasive and reliable imaging modality allowing to detect early signs of asbestosis and mesothelioma development such as pleural effuson, pleural thickening and nodules. LUS can play a main role in the follow-up of these pathological findings or to monitor subjects with referred asbestos exposure. Moreover, ultrasound seems to be an optimal choice to guide pleural fluid sampling or mass biopsies in order to perform biochemical and cytological examination [15, 36, 37]. Therefore, in our opinion, LUS is nowadays an undeniable tool to make MPM diagnosis and to guide its clinical and therapeutical management.
Funding: This study was no funded.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Soldati, G., and Demi, M. (2017). The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. Journal of ultrasound 20, 91-96.
- Zanforlin, A., Smargiassi, A., Inchingolo, R., Valente, S., and Ramazzina, E. (2015). Ultrasound in obstructive lung diseases: the effect of airway obstruction on diaphragm kinetics. A short pictorial essay. Journal of ultrasound 18, 379-384.
- Vitturi, N., Soattin, M., Allemand, E., Simoni, F., and Realdi, G. (2011). Thoracic ultrasonography: A new method for the work-up of patients with dyspnea(). Journal of ultrasound 14, 147-151.
- De Luca, C., Valentino, M., Rimondi, M.R., Branchini, M., Baleni, M.C., and Barozzi, L. (2008). Use of chest sonography in acute-care radiology(). Journal of ultrasound 11, 125-134.
- D’Angelo, A., De Simone, C., Pagnottella, M., Rossi, S., Pepe, R., Ruggieri, G., Cocco, G., and Schiavone, C. (2017). A case of Legionella pneumophila evaluated with CT and ultrasound. Journal of ultrasound 20, 243-245.
- Lichtenstein, D.A., and Meziere, G.A. (2008). Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 134, 117-125.
- Lichtenstein, D.A., Meziere, G., Lascols, N., Biderman, P., Courret, J.P., Gepner, A., Goldstein, I., and Tenoudji-Cohen, M. (2005). Ultrasound diagnosis of occult pneumothorax. Critical care medicine 33, 1231-1238.
- Kruisselbrink, R., Chan, V., Cibinel, G.A., Abrahamson, S., and Goffi, A. (2017). I-AIM (Indication, Acquisition, Interpretation, Medical Decision-making) Framework for Point of Care Lung Ultrasound. Anesthesiology 127, 568-582.
- Sperandeo, M., Filabozzi, P., Varriale, A., Carnevale, V., Piattelli, M.L., Sperandeo, G., Brunetti, E., and Decuzzi, M. (2008). Role of thoracic ultrasound in the assessment of pleural and pulmonary diseases. Journal of ultrasound 11, 39-46.
- Soldati, G., Smargiassi, A., Mariani, A.A., and Inchingolo, R. (2017). Novel aspects in diagnostic approach to respiratory patients: is it the time for a new semiotics? Multidisciplinary respiratory medicine 12, 15.
- Samanta, S., Singh, R.K., Baronia, A.K., Poddar, B., Azim, A., and Gurjar, M. (2017). Diaphragm thickening fraction to predRegarding the values of PaO2/FiO2 rati and Δ(A-a)O2, the statistical analysis instead showed a significant difference compared to initial values, even if in a later phase (see Table 2).ict weaning-a prospective exploratory study. Journal of intensive care 5, 62.
- Saporito, A., Lo Piccolo, A., Franceschini, D., Tomasetti, R., and Anselmi, L. (2013). Thoracic ultrasound confirmation of correct lung exclusion before one-lung ventilation during thoracic surgery. Journal of ultrasound 16, 195-199.
- Smargiassi, A., Pasciuto, G., Pedicelli, I., Lo Greco, E., Calvello, M., Inchingolo, R., Schifino, G., Capoluongo, P., Patriciello, P., Manno, M., et al. (2017). Chest ultrasonography in health surveillance of asbestos-related lung diseases. Toxicology and industrial health 33, 537-546.
- Scarlata, S., Finamore, P., Giannunzio, G., Santangelo, S., and Antonelli Incalzi, R. (2017). Chest ultrasonography in health surveillance of asbestos related pleural disease. Lung cancer (Amsterdam, Netherlands) 111, 139-142.
- (2007). BTS statement on malignant mesothelioma in the UK, 2007. Thorax 62 Suppl 2, ii1-ii19.
- Beckett, P., Edwards, J., Fennell, D., Hubbard, R., Woolhouse, I., and Peake, M.D. (2015). Demographics, management and survival of patients with malignant pleural mesothelioma in the National Lung Cancer Audit in England and Wales. Lung cancer (Amsterdam, Netherlands) 88, 344-348.
- Scherpereel, A., Astoul, P., Baas, P., Berghmans, T., Clayson, H., de Vuyst, P., Dienemann, H., Galateau-Salle, F., Hennequin, C., Hillerdal, G., et al. (2010). Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. The European respiratory journal 35, 479-495.
- Lopez-Rios, F., Illei, P.B., Rusch, V., and Ladanyi, M. (2004). Evidence against a role for SV40 infection in human mesotheliomas and high risk of false-positive PCR results owing to presence of SV40 sequences in common laboratory plasmids. Lancet 364, 1157-1166.
- van Zandwijk, N., Clarke, C., Henderson, D., Musk, A.W., Fong, K., Nowak, A., Loneragan, R., McCaughan, B., Boyer, M., Feigen, M., et al. (2013). Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. Journal of thoracic disease 5, E254-307.
- Roberts, M.E., Neville, E., Berrisford, R.G., Antunes, G., and Ali, N.J. (2010). Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 65 Suppl 2, ii32-40.
- Qureshi, N.R., Rahman, N.M., and Gleeson, F.V. (2009). Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 64, 139-143.
- Bibby, A.C., and Maskell, N.A. (2016). Pleural biopsies in undiagnosed pleural effusions; Abrams vs image-guided vs thoracoscopic biopsies. Current opinion in pulmonary medicine 22, 392-398.
- Bandi, V., Lunn, W., Ernst, A., Eberhardt, R., Hoffmann, H., and Herth, F.J. (2008). Ultrasound vs. CT in detecting chest wall invasion by tumor: a prospective study. Chest 133, 881-886.
- McLoud, T.C., and Flower, C.D. (1991). Imaging the pleura: sonography, CT, and MR imaging. AJR. American journal of roentgenology 156, 1145-1153.
- Chian, C.F., Su, W.L., Soh, L.H., Yan, H.C., Perng, W.C., and Wu, C.P. (2004). Echogenic swirling pattern as a predictor of malignant pleural effusions in patients with malignancies. Chest 126, 129-134.
- Yang, P.C., Luh, K.T., Chang, D.B., Wu, H.D., Yu, C.J., and Kuo, S.H. (1992). Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR. American journal of roentgenology 159, 29-33.
- Davies, H.E., Mishra, E.K., Kahan, B.C., Wrightson, J.M., Stanton, A.E., Guhan, A., Davies, C.W., Grayez, J., Harrison, R., Prasad, A., et al. (2012). Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. Jama 307, 2383-2389.
- Bibby, A.C., Gibbs, L., and Braybrooke, J.P. (2015). Medical and oncological management of malignant mesothelioma. British journal of hospital medicine (London, England : 2005) 76, 384-389.
- Gordon, C.E., Feller-Kopman, D., Balk, E.M., and Smetana, G.W. (2010). Pneumothorax following thoracentesis: a systematic review and meta-analysis. Archives of internal medicine 170, 332-339.
- Diacon, A.H., Brutsche, M.H., and Soler, M. (2003). Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest 123, 436-441.
- Mayo, P.H., and Doelken, P. (2006). Pleural ultrasonography. Clinics in chest medicine 27, 215-227.
- Diacon, A.H., Theron, J., Schubert, P., Brundyn, K., Louw, M., Wright, C.A., and Bolliger, C.T. (2007). Ultrasound-assisted transthoracic biopsy: fine-needle aspiration or cutting-needle biopsy? The European respiratory journal 29, 357-362.
- DiBardino, D.M., Yarmus, L.B., and Semaan, R.W. (2015). Transthoracic needle biopsy of the lung. Journal of thoracic disease 7, S304-316.
- Jarmakani, M., Duguay, S., Rust, K., Conner, K., and Wagner, J.M. (2016). Ultrasound Versus Computed Tomographic Guidance for Percutaneous Biopsy of Chest Lesions. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 35, 1865-1872.
- Wang, J., Zhou, X., Xie, X., Tang, Q., Shen, P., and Zeng, Y. (2016). Combined ultrasound-guided cutting-needle biopsy and standard pleural biopsy for diagnosis of malignant pleural effusions. BMC pulmonary medicine 16, 155.
- Renshaw, A.A., Dean, B.R., Antman, K.H., Sugarbaker, D.J., and Cibas, E.S. (1997). The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest 111, 106-109.
- Hooper, C., Lee, Y.C., and Maskell, N. (2010). Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 65 Suppl 2, ii4-17.