- Pietro Pozzessere
- Original Article
Late-onset deficit of ornithine transcarbamylase: a case report and review of the literature
- 2/2019-giugno
- ISSN 2532-1285
- https://doi.org/10.23832/ITJEM.2019.021
Pietro Pozzessere1, Michela Nardacci2, Mariangela Portaluri2, Serafina Schiraldi2, Vito Procacci1
1) Department of Emergency Medicine and Surgery “Azienda Ospedaliera Consorziale Policlinico” of Bari, Italy
2) Postgraduate school in Emergency Medicine University of Bari Medical School Bari, Italy
Abstract
Introduction
Case report
Conclusion
It is necessary to always consider ammonia levels in adult patients affected by unexplained coma. The correlation between plasma ammonium concentration and hepatic encephalopathy is not consistent, thus suggesting that other factors are important in producing brain edema. Case reports like this are important because they show that the late phenotypic expression of OCT deficiency carriers is the result of the interactions between environmental conditions and genetic anomalies.
Keywords
Hyperammonaemia, Ornithine transcarbamylase deficiency, late-onset defect of urea cycle
Introduction
- Inherited dfects of the urea cycle
- Secondary urea cycle disturbance 1 – Inherited: amino acid transporter defects
- Inherited defects of fatty acid oxidation
- Inherited organic acid disorders
- Hypovolaemic shock, congestive cardiac failure
- Circulatory abnormalities with portosystemic shunting
- Medications; anticonvulsants: sodiumvalproate, topiramate (carbamazepine?); chemotherapeutic drugs: asparaginase, 5-fluorouracil; salicylates (with other predisposing factors)
- Haematological malignancies: multiple myeloma, leukaemia
- Urinary infection with urease-producing bacteria
- Excessive amino acid load/increased catabolism: gastrointestinal haemorrhage, glycine irrigation, cachexia+high protein feeds
Case Report
A 21-years-old white man, previously healthy, was admmittedto the Emergency Room following a new-onset in a few hours of slurred speech and nausea.
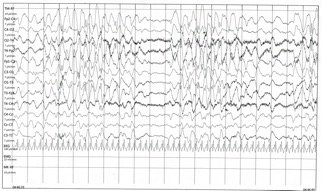
Figure 1. This electroencephaloghraphy portion of long-term tracing demonstrates background slowing in the range of 3HZ with brief intervals characterized by reduction of electric activity
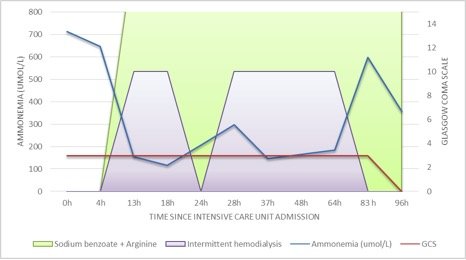
Urea cycle disorder was strongly suspected and so hemodialysis (CVVHDF) was initiated as well as plasma and urine amino acid analysis and urine organic acid quantitation were performed. After the second hemodialysis session we witnessed a transient progressive reduction of ammonia level without any observable improvement in his level of consciousness.
The blood test showed high level of glutamine, ornithine, arginine, lysine and normal level of citrulline, while the urine test revealed elevated levels of orotic acid (4,4 uM/M creatinine; v.n.<0,29). A liver biopsy was performed for genetic testing. The transcranic color doppler ultrasound, performed two days after the admission in ICU, certified absence of cerebral flow and four days later the patient was declared dead. The genetic testing was performed post-mortem by the CoNVaDING tool and confirmed by multiplex ligation-dependent probeamplification (MLPA).
The sequence analysis of OTC gene showed hemizygosis for pathological variant known as c.392T>Cp.(Leu131Ser) of OCT gene.
Discussion
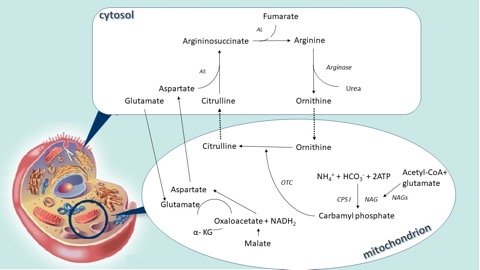
The urea cycle is the main way through which the ammonium and nitrogen in excess bound to the amino groups are converted into urea and then excreted in the urine. It is the result of 6 consecutive reactions, three mitochondrial and three in the cytosol, and at the end of each cycle two nitrogen atoms are converted into urea, one that comes from ammonium and another from the amino acid pool, with the alanine as the main component, both channelled through aspartate.
Conclusions
Bibliografia
-
P. Colombo, E. Peheim, R. Kretschmer et al. Plasma ammonia concentrations in newborns and children Clinica Chimica Acta 1984; 138 (3) 283-91
-
Romero-Gómez, M. Jover, J. J. Galá et al. Gut ammonia production and its modulation, Metab. Brain Dis. 2009; 24:147–157
-
Jungermann K. Functional heterogeneity of periportal and perivenous hepatocytes. Enzyme 1986; 35:161–80
-
Bosoi CR, Rose CF. Identifying the direct effects of ammonia on the brainMetab Brain Dis 2009; 24:95–102
-
Natesan V, Mani R, Arumugam R. Clinical aspects of urea cycle dysfunction and altered brain energy metabolism on modulation of glutamate receptors and transporters in acute and chronic hyperammonemia Biomed Pharmacother 2016 Jul 81:192-202
-
Häberle, N. Boddaert, A. Burlina, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders Orphanet J. Rare Dis. 2012; 29:7-32
-
White LP, Phear EA, Summerskill WH, et al. Ammonium tolerance in liver disease: observations based on catheterization of the hepatic veins.J Clin Invest 1955; 34:158–68
-
Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med 2003; 114:188–93
-
Bachmann, in: C. Bachmann, J. Häberle, Eds.), Pathophysiology and Management of Hyperammonemia, SPS Publications, Heilbronn, 2006
-
Uchino, F. Endo, I. Matsuda et al.: Neurodevelopmental outcome of long-termtherapyof urea cycle disorders in Japan Metab. Dis.1998; 21 (Suppl. 1) 151-59
-
Häberle, Clinical and biochemical aspects of primary and secondary hyperammonemic disorders Archives of Biochemistry and Biophysics 2013; 536:101–108
-
S. Brusilow, A. Horwich, in: C. Scriver, A. Beaudet, W. Sly, D. Valle (Eds.), The Metabolic and Molecular Bases of Inherited Disease, McGraw-Hill, New York, 2001
-
J. Häberle,Clinical practice: The management of hyperammoniemia Eur. J. Pediatr. 2011; 170:21–34
-
P. M. Stewart, M. Walser Failure of the normal ureagenic response to amino acids in organic acid-loaded rats. Proposed mechanism for the hyperammonemia of propionic and methylmalonicacidemia. J. Clin. Invest. 1980; 66:484–492
-
G.A. Mitchell, N. Gauthier, A. Lesimple, Hereditary and acquired diseases of acyl-coenzyme A metabolism Mol. Genet. Metab. 2008; 94 1: 4-15
-
C. Jorck, W. Kiess, J. F. Weigel, U. Mutze et al.Transienthyperammonemia due to L-asparaginase therapy in children with acute lymphoblastic leukemia or non-Hodgkin lymphoma Pediatr. Hematol. Oncol. 2011; 28:3–9
-
S. Kapila, M. Saba, C.H. Lin, E.V. Bawle Arginine deficiency-induced hyperammonemia in a home total parenteral nutrition-dependent patient: a case report, J pen 2001; 25:286–288
-
J. Häberle, B. Görg, F. Rutsch, Congenital glutamine deficiency with glutamine synthetase mutations New Engl. J. Med. 353 (2005) 1926–1933
-
G. R. Lichtenstein, Y. X. Yang, F. A. Nunes, Fatal hyperammonemia after orthotopic lung transplantation Ann. Intern. Med.2000; 132:283–287
-
T. Taguchi, S. Iwamura, M. Mizobuchi, Y. Terada, Hepatic arteriovenous malformation with hyperammonemia in Rendu-Osler-Weber syndrome J. Gastrointest. Liver Dis. 2011; 20:330–331
-
M. Tuchman, M.K. Georgieff, Transient hyperammonemia of the newborn: a vascular complication of prematurity? J. Perinatol. 1992; 12:234–236
-
Kinne-Saffran E, Kinne RK Vitalism and synthesis of urea. From Friedrich Wöhler to Hans A. Krebs.Am JNephrol, 1999; 19: 290–94
-
Walker V. Ammonia toxicity and its prevention in inherited defects ofthe urea cycle. Diabetes Obes Metab 2009; 11:823–35
-
Maria M. Adevaa, Gema Souto, N. Blanco Ammonium metabolism in humans Metabolism clinical and experimental 61 (2012) 1495–1511
-
ME Jones, A.D. Anderson, C. Anderson et al. Citrulline synthesis in rat tissues, Arch. Biochem. Biophys. 95 (1961) 499–507
-
Vijayakumar Natesan, Renuka Mani, Ramakrishnan Arumugam Clinical aspects of urea cycle dysfunction and altered brain energy metabolism on modulation of glutamate receptors and transporters in acute and chronic hyperammonemia Biomedicine & Pharmacotherapy 81 (2016) 192–202
-
Valerie Walker Severe hyperammonaemia in adults not explained by liver disease Ann Clin Biochem 2012; 49: 214–228
-
Marshall L. Summar, Stefan Koelker, Debra Freedenberg The incidence of urea cycle disorders Molecular Genetics and Metabolism 110 (2013) 179–180
-
Gordon N. Ornithine transcarbamylase deficiency: a urea cycle defect. Eur J PaediatrNeurol 2003; 7:115–21
-
Lien J, NyhanWL, Barshop BA. Fatal initial adult-onsetpresentation of urea cycle defect. Arch Neurol 2007; 64:1777–9
-
Marshall L Summar, Dries Dobbelaere 2, Saul Brusilow Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes Acta Pædiatrica 2008; 97, pp. 1420–1425
-
Uchino T, Endo F, Matsuda I. Neurodevelopmental outcome of long-term therapy of urea cycle disorders in Japan. J Inherit Metab Dis 1998; 21 Suppl 1: 151–9
-
Yamaguchi S, Brailey LL, Morizono ET AL.: Mutations and polymorphisms in the human ornithine transcarbamylase (OTC) gene. Hum Mutat 2006; 27:626–632
-
Msall M, Batshaw ML, Suss R, et al. Neurologic outcomein children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med 1984; 310:1500–5
-
Teufel U, Weitz J, Flechtenmacher C. et al. High urgency liver transplantation in ornithinetranscarbamylase deficiency presenting with acute liver failure. Pediatr Transplant 2011; 15:E110–E115
-
Gallagher RC, Lam C, Wong D et al. Significant hepatic involvement in patientswith ornithine transcarbamylase deficiency. J Pediatr. 2014; 164(4):720–5 e 6
-
M. L. Batshaw, M Tuchman, M Summar A longitudinal study of urea cycle disorders Molecular Genetics and Metabolism 113 (2014) 127–130
-
Summar ML, Barr F, Dawling S, et al. Unmasked adult-onset urea cycle disorders in the critical care setting. Crit Care Clin 2005; 21:S1–S8
-
Arranz JA, Riudor E, Marco-Marin C, Estimation of the total number of disease-causing mutations in ornithine transcarbamylase (OTC) deficiency. Value of the OTC structure in predicting a mutation pathogenic potential. J Inherit Metab Dis 2007; 30:217–226
-
L. Krivitzky, T. Babikian, H.S. Lee, et al. Intellectual, adaptive, and behavioral functioning in children with urea cycle disorders. Pediatr. Res. 66 (2009) 96–101
-
Andrea L. Gropman and Mark L. Batshaw Cognitive outcome in urea cycle disorders Molecular Genetics and Metabolism 81 (2004) S58–S62
-
M. L. Batshaw, Hyperammonemia, Curr. Probl. Pediatr. 14 (1984)1–69
-
E. Drogari, J.V. Leonard, Late onset ornithine carbomyltransferase deficiency in males, Arch. Dis. Child. 63 (1988)1363–1367
-
M. C. Nassogne, B. He´ Ron, G. Touati Urea cycle defects: Management and outcome J. Inherit. Metab. Dis. 28 (2005) 407-414
-
Z Ben-Ari, A Dalal, A Morry Adult-onset ornithine transcarbamylase (OTC) deficiency unmasked by the Atkins’ diet Journal of Hepatology 2010 vol. 52 j 292–295
-
H Singh, G Babu Nanjundappa, S Kumar et al. Carbamazepine Induced Asterixis with Hyperammonemia: A Case Report with Review of Literature Indian Journal of Psychological Medicine| Jan – Mar 2015 | Vol 37 | Issue 1 99-101
-
Ambrosetto G, Riva R, Baruzzi A. Hyperammonemia in asterixis induced by carbamazepine: two case reports. Acta Neurol Scand. 1984; 69:186-9
-
Rivelli M, El-Mallakh RS, Nelson WH. Carbamazepine-associated asterixis and hyperammonemia. Am J Psychiatry. 1988; 145:269-70
-
EN. Adams, A Marks, and MH. Lizer Carbamazepine-induced hyperammonemia Am J Health-Syst Pharm Vol 66 Aug 15, 2009
-
Neuvonen PJ. Bioavailability and central side effects of different carbamazepine tablets. Int J Clin Pharmacol Ther Toxicol 1985; 23:226–32
-
Tothfalusi, S. Speidl, L. Endrenyi et. al: Exposure–response analysis reveals that clinically important toxicity difference can exist between bioequivalent carbamazepine tablets. Br J Clin Pharmacol 2007; 65:1/110–122
-
NM Jaramillo, IF Galindo, AO Vázquez Pharmacogenetic potential biomarkers for carbamazepine adverse drug reactions and clinical response. Drug Metab Drug Interact 2014; 29(2): 67–79
-
C. Lewis, A. Deshpande, G. E. Tesar, et al. Valproate-induced hyperammonaemic encephalopathy: a brief review Curr. Med. Res. Opin. 28 (2012) 1039–1042
-
Aires CC, van Cruchten A, Ijlst L, et al: New insights on the mechanisms of valproate-induced hyperammonemia: Inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol 2011; 55:426–434
-
M. J. Dealberto, Valproate-induced hyperammonaemic encephalopathy: review of 14 cases in the psychiatric setting, Int. Clin. Psychopharmacol. 22 (2007) 330–337
-
Xiaopeng Guo, Lu Gao Wei Lin et al. Hyperammonemia induced by prophylactic administration of antiepileptic drugs during the perioperative period of craniotomy. Clinica Chimica Acta 462 (2016) 33–39
-
McCormack M, Alfirevic A, Bourgeois S, HLA-A*3101 and carbamazepine induced hypersensitivity reactions in Europeans. N Engl J Med 2011; 364:1134–43
-
Man C, Kwan P, Baum L, Yu E,et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia 2007; 48:1015–8
-
Brunquell P, Tezcan K, DiMario FJ Jr:Electroencephalographic findings in ornithine transcarbamylase deficiency. J Child Neurol 14:533-536, 1999
-
Amodio P, Marchetti P, Del Piccolo F et al: Spectral versus visual EEG analysis in mild hepatic encephalopathy. Clin Neurophysiol, 1999; 110:1334–44
-
Bathla G, Hegde AN: MRI and CT appearances in metabolic encephalopathies due to systemic diseases in adults. Clin Radiol, 2013; 68:545–54
-
Bindu PS, Sinha S, Taly AB, et al: Extensive cortical magnetic resonancesignal change in proximal urea cycle disorder. J Child Neurol 22:238-239, 2007
-
Gregory M. Enns Neurologic Damage and Neurocognitive Dysfunction in Urea Cycle Disorders Semin Pediatr Neurol 15:132–139 © 2008
-
Johannes Häberle Clinical and biochemical aspects of primary and secondary Iperammonemic disorders Archives of Biochemistry and Biophysics 536 (2013) 101–108
-
Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr 1996; 43:127-70
-
D E Choi, K W Lee, Y T Shin Hyperammonemia in a Patient with Late-Onset Ornithine Carbamoy ltransferase Deficiency J Korean Med Sci 2012; 27:556-559
-
Shchelochkov OA, Li FY, Geraghty MT, Gallagher RC, et al: High-frequency detection of deletions and variable rearrangements at the ornithine transcarbamylase (OTC) locus by oligonucleotide array CGH. Mol Genet Metab 2009; 96:97–105
-
Tuchman M:Allopurinol-induced orotidinuria. N Engl J Med 1990; 323:1352-1353
-
Potter M, Hammond JW, SimKG, et al.: Ornithinecarbamoyltransferase deficiency: improved sensitivity of testing forprotein tolerance in the diagnosis of heterozygotes. J Inherit Metab Dis 2001; 24:5–14
-
L Caldovic, I Abdikarim, S NarainGenotype–Phenotype Correlations in Ornithine Transcarbamylase Deficiency: A Mutation Update J Genet Genomics. 2015 May 20; 42(5): 181–194
-
F. Butterworth, Hepatic encephalopathy, Alcohol Res. Health 27 (2003) 240–246
-
Olivier Braissant Current concepts in the pathogenesis of urea cycle disorders Molecular Genetics and Metabolism 100 (2010) S3–S12
-
Batshaw ML, Brusilow SW. Evidence of lack of toxicity of sodium phenylacetate and sodium benzoate in treating urea cycle enzymopathies. J Inherit Metab Dis1981; 4(4):231
-
Batshaw ML, Brusilow SW. Treatment of hyperammonaemic coma caused by inborn errors of urea synthesis. J Pediatr 1980; 97(6):893– 900
-
BIMDG_Adult_UCD_Revision2018
-
Hiroma T, Nakamura T, Tamura M et al.Continuous venovenoushemodiafiltration in neonatal onset hyperammonemia. Am J Perinatol 2002; 19:221–224
-
CY Chen, YC Chen, Ji-Tseng Fang et al. Continuous arteriovenoushemodiafiltration in the acute treatment of hyperammonaemia due to ornithine transcarbamylase deficiency Ren failure, 22:6, 823-836 (2000)
-
Chang MY, Fang JT, Chen YC, Huang CC: Continuous venovenous hemofiltration in htperammonaemic coma of an adult with non-diagnosed partial ornithine transcarbamylase deficiency. Nephrol Dial transplant 14: 1282-1284,1999.